Chemodenervation
Injecting Botulinum neurotoxin (BoNT) into the affected musculature has proved to be the most effective means of managing conditions such as cervical and oromandibular dystonia. [1] [2] Chemodenervation with BoNT has become the management modality of choice for patients with oromandibular dystonia due to its high efficacy albeit temporary relief.
Eight serologically distinct subtypes of BoNT (A, B, C1, C2, D, E, F and G) have been isolated, of which, subtypes A and B are approved by FDA for patients with motor disorders.
Botulinum neurotoxin type A (BoNT/A) is the most potent and longest acting (8 to 16 weeks), [3] and is used off-label in the orofacial region to help treat primary and secondary masticatory and facial muscle spasm, severe bruxism, facial tics, orofacial dyskinesias, dystonias, focal facial dystonia, and even idiopathic hypertrophy of the masticatory muscles. [4] [5]
Like what you’re learning? Test your diagnosis skills with USC’s Virtual Patient Simulation. Review real-life patient histories, conduct medical interviews and exams, make a diagnosis, and create a treatment plan for patients experiencing orofacial pain.

Benefits of Botox for Oromandibular Dystonia
The therapeutic benefit of BoNT is mainly due to its primary action of blocking the release of acetylcholine into the neuromuscular junction. More specifically, BoNT achieves this effect by cleaving SNARE proteins that are required for the docking of the ACh vesicle to the presynaptic membrane. [6]
In case you missed it: read part 1 of this series: sensory tricks and medical management.
Immunoresistance
Repeated injections of BoNT/A has been reported to cause immunoresistance or development of antibodies against the toxin, rendering it ineffective in some patients. [7] This is a frustrating outcome for both the patient and the practitioner.
A simple clinical test to determine whether a patient may be resistant to BoNT is to inject a small amount of BoNT (20 U of BoNT/A or 1000 U of BoNT/B) unilaterally into one corrugator-procerus muscle complex. A lack of frowning due to weakness of the injected muscles is indicative of lack of immunoresistance and is referred to as the unilateral brow injection test. [8]
Like what you’re learning? Download a brochure for our Orofacial Pain and Oral Medicine certificate or master’s degree program.
Side Effects of Botox Injections for Dystonia
Contraindications to the use of botulinum toxin include allergy to the drug, infection or inflammation at the injection site, pregnancy, women who are lactating, inability of the patient to cooperate and high levels of fearfulness toward the method.
Side effects of BoNT injections can be divided into site-of-injection side effects and medication-related side effects.
Site-of-Injection Side Effects
Site-of-injection side effects are rare and include local hematoma, infection or persistent pain in the injection site. These complications are usually a result of injecting into infected or nonsterile skin.
Medication-Related Side Effects
The medication-related side effects are transient and include muscle weakness (e.g. weakening of the muscles of facial expression or swallowing), and tremor. [9]
Lateral pterygoid muscle injections or palatal muscle injections may result in slurred speech and palatal weakness. Infrequently, patients may experience a thickening of the saliva, a desirable side effect in those with excessive salivation.
Botulinum B has been specifically associated with an increased risk of adverse effects such as sore throat and dry mouth. [10]
Chemodenervation Injection
Blitzer et al (1989) first described the injection of BoNT-A for oromandibular dystonia. [11] In their article, they described injecting many of the orofacial muscles in 20 oromandibular dystonia patients and claimed that masseter and temporalis injections helped with suppressing the overall oromandibular dystonia.
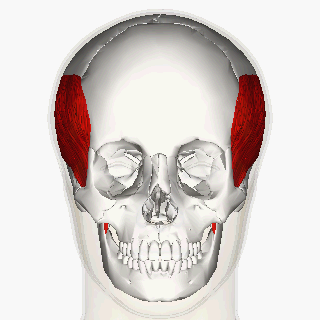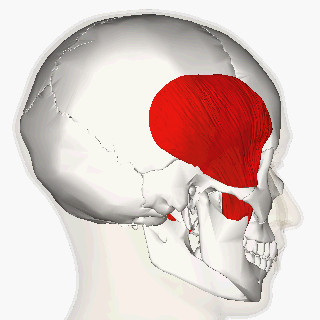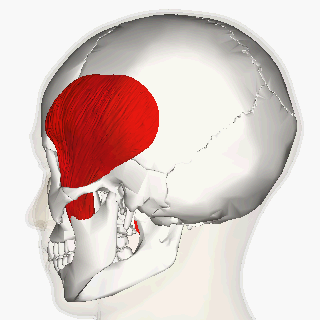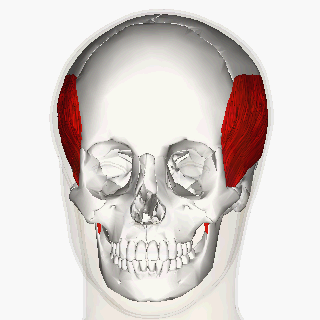
More recent reports indicate a moderate improvement of oral dystonia in two-third of the patients treated with BoNT-A, mainly in the conditions when the jaw closes. [12]
In the case of an involuntary jaw-opening dystonia, one complication is that the temporomandibular joint can become physically locked in the wide-open position so that even after the dystonic contraction stops, the jaw will not easily close.
Moore and Wood (1997) described the treatment of recurrent, involuntary TMJ dislocation using BoNT-A. [13] The injected target was the lateral pterygoid muscle and the injection was performed using electromyographic guidance. The authors describe that the effect lasted for 10 months. This same approach has been successfully used with children with recurrent TMJ dislocation. [14]
The lateral pterygoid is the muscle most responsible for opening and it is a difficult injection, which has a high potential for misplacement of the solution into other adjacent muscles.
The long-term management of oromandibular dystonia with botulinum toxin injecting temporalis, masseter or/and lateral pterygoid muscles has shown minimal morbidity being a useful approach for this disorder. [15]
Earn an Online Postgraduate Degree in Orofacial Pain and Oral Medicine
Are you interested in a variety of issues focused on orofacial pain, medicine and sleep disorders? Consider enrolling in the Herman Ostrow School of Dentistry of USC’s online, competency-based certificate or master’s program in Orofacial Pain and Oral Medicine.
References
[1] Clark GT, Ram S. Four oral motor disorders: bruxism, dystonia, dyskinesia and drug-induced dystonic extrapyramidal reactions. Dent Clin North Am 2007;51(1):225-43.
[2] Castelão, M., Marques, R., Duarte, G., Rodrigues, F., Ferreira, J., Sampaio, C., Costa, J. (2017). Botulinum toxin type A therapy for cervical dystonia. The Cochrane Database of Systematic Reviews., 12, CD003633.
[3] Nigam, P K, & Nigam, Anjana. (2010). Botulinum toxin.Indian Journal of Dermatology., 55( 1), 8-14.
[4] Clark GT, Stiles A, Lockerman LZ et al. A critical review of the use of botulinum toxin in orofacial pain disorders. Dent Clin North Am 2007;51(1):245-61.
[5] Ruiz-de-León-Hernández, G., Díaz-Sánchez, R., Torres-Lagares, D., Hernández-Pacheco, E., González-Martín, M., & Serrera-Figallo, M. (2017). Botulinum toxin A for patients with orofacial dystonia: Prospective, observational, single-centre study. International Journal of Oral & Maxillofacial Surgery.,International journal of oral & maxillofacial surgery. , 2017.
[6] Chen S, Kim JJ, Barbieri JT. Mechanism of substrate recognition by botulinum neurotoxin serotype A. J Biol Chem 2007;282(13):9621-7.
[7] Adler CH, Factor SA, Brin M et al. Secondary nonresponsiveness to botulinum toxin type A in patients with oromandibular dystonia. Mov Disord 2002;17(1):158-61.
[8] Jankovic J. Treatment of dystonia. Lancet Neurol 2006;5(10):864-72.
[9] Raoofi, S., Khorshidi, H., & Najafi, M. (2017.). Etiology, Diagnosis and Management of Oromandibular Dystonia: An Update for Stomatologists. Journal of Dentistry., 18(2), 73-81.
[10] Duarte, G., Castelão, M., Rodrigues, F., Marques, R., Ferreira, J., Sampaio, C., Moore A. Costa, J. (2016). Botulinum toxin type A versus botulinum toxin type B for cervical dystonia. The Cochrane Database of Systematic Reviews., 10, CD004314.
[11] Blitzer A, Brin MF, Greene PE, Fahn S. Botulinum toxin injection for the treatment of oromandibular dystonia. Ann Otol Rhinol Laryngol 1989 Feb;98(2):93-7.
[12] Karp, B., & Alter, K. (2016). Botulinum Toxin Treatment of Blepharospasm, Orofacial/Oromandibular Dystonia, and Hemifacial Spasm. Seminars in Neurology., 36(1), 84-91.
[13] Moore AP; Wood GD. Medical treatment of recurrent temporomandibular joint dislocation using botulinum toxin A. British Dental Journal, 183(11-12):415-7, 1997.
[14] Stark, T., Perez, C., & Okeson, J. (2015). Recurrent TMJ Dislocation Managed with Botulinum Toxin Type A Injections in a Pediatric Patient. Pediatric Dentistry., 37(1), 65-69.
[15] Sinclair, C., Gurey, L., & Blitzer, A. (2013). Oromandibular dystonia: Long-term management with botulinum toxin. The Laryngoscope, 123(12), 3078-3083.

