Introduction
Sleep-disordered breathing (SDB) in children has been recognized and treated since the 1800s with adenotonsillectomy (AT surgery) [1]. What once might have been dismissed as simple snoring is now understood as a serious health concern with potential lifelong consequences. Proper sleep is critically important for children’s development, with disturbances linked to increased risk of cardiac, metabolic, and neurocognitive issues that can persist into adulthood [2, 3]. Children with obstructive sleep apnea (OSA) utilize healthcare resources 2.3 times more frequently than their peers [2, 3], highlighting both the health and economic implications of this condition.
The rising prevalence of childhood obesity shows a bidirectional relationship with sleep apnea, each condition potentially exacerbating the other [4, 2, 1]. The disturbed sleep and nocturnal hypoxia associated with SDB can initiate pathophysiological cascades resulting in left ventricular hypertrophy and childhood hypertension, potentially leading to significant cardiovascular morbidity decades later [5, 6]. As dental professionals, we occupy a privileged position to identify early warning signs during routine examinations of orofacial structures [7, 8].
Epidemiology of Pediatric SDB
According to the European Respiratory Society [9], obstructive SDB represents a spectrum of airway dysfunctions that may progress from primary snoring to full OSA (Figure 1).
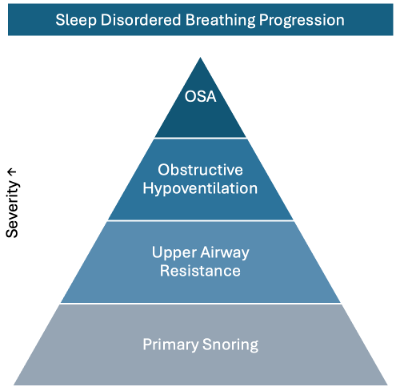
Figure 1. The Progression Spectrum of Sleep-Disordered Breathing: From primary snoring to upper airway resistance syndrome to obstructive sleep apnea, illustrating the potential linear progression of respiratory disturbance during sleep in pediatric patients.
SDB in childhood typically peaks between ages 2-8 due to disproportionate lymphoid tissue growth compared to airway development. Among overweight children aged 10-16, prevalence ranges from 13-39%, reflecting the concerning rise in childhood obesity rates [10, 11, 4]. Cases in children under 2 years are typically associated with syndromic, neuromuscular, or congenital factors [4].
The overall prevalence of primary snoring is approximately 7.45% (range 3-35%), while pediatric OSA affects 1-4% of children (range 0.1-13%). Of clinical significance, 2-3% of children diagnosed with primary snoring will develop clinical OSA, underscoring the importance of careful evaluation [4]. Known risk factors include craniofacial abnormalities, asthma, obesity, premature birth, Down syndrome, African American ethnicity, tobacco exposure, low socioeconomic status, facial trauma history, and untreated allergic rhinitis [9, 4].
Like what you’re learning? Download a brochure for our Orofacial Pain and Oral Medicine certificate or master’s degree program.
Clinical Symptoms and Diagnosis
Dental practitioners and healthcare practitioners should be alert to signs of adenotonsillar hypertrophy [4] and associated malocclusions, including posterior crossbite [12, 13], increased vertical dimension, retrognathia, steep mandibular plane, and macroglossia. Sleep bruxism merits particular attention, as it may present in up to 50% of children with SDB [15]. These are important risk factors and clinical symptoms (Figure 2).
Parental reports often include snoring, mouth breathing, bedwetting, frequent night-time arousals, daytime sleepiness, and witnessed apneas [16]. Associated symptoms include nasal congestion and allergic rhinitis, the latter increasing OSA risk threefold [17, 4]. Behavioral manifestations range from exhaustion to hyperexcitability, aggression, ADHD-like symptoms, and cognitive difficulties [9].
Definitive diagnosis requires polysomnography (PSG) with pediatric-specific scoring criteria: mild (1-4.9 events/hr), moderate (5-9.9 events/hr), and severe (>10 events/hr) [16]. Treatment is generally indicated when the apnea-hypopnea index (AHI) exceeds 5 events/hour, as spontaneous resolution becomes unlikely at this threshold [9].
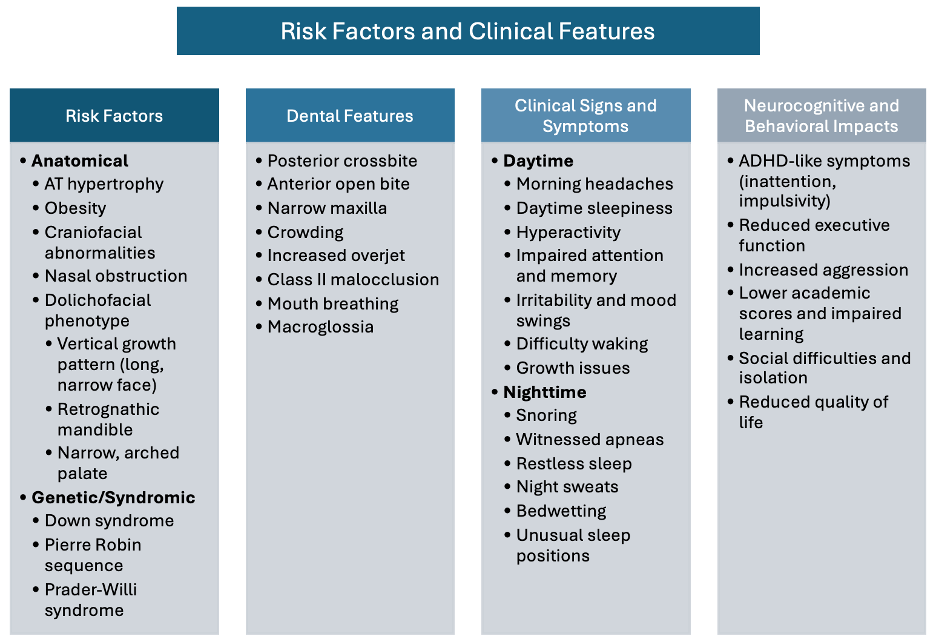
Figure 2. Comprehensive Clinical Assessment Framework: Key risk factors and diagnostic indicators for pediatric sleep-disordered breathing, including craniofacial features, sleep behaviors, and comorbid conditions that should trigger further evaluation.
Treatment Options
Management approaches vary based on severity and associated factors (Figure 3). Mild-moderate pediatric OSA may respond to pharmacological interventions such as montelukast or intranasal steroids [16]. Improved sleep hygiene and watchful waiting can lead to normalized results in up to 46% of cases, particularly given that adenoid tissue naturally decreases in size beginning around age 10 [16].
For cases with significant adenotonsillar hypertrophy, the American Academy of Otolaryngology recommends tonsillectomy [18]. AT surgery results in significant improvement in more than 70% of otherwise healthy children, though up to 70% of children with additional risk factors may experience residual OSA following surgery [19].
Additional interventions include CPAP, BiPAP, and myofunctional therapy [20]. Orthodontic approaches, particularly rapid maxillary expansion (RME), can effectively increase nasal airway size while reducing adenoid and tonsil volume by up to 51.6% and 75.4% respectively [21, 8, 20]. Post-treatment polysomnography is essential due to the high risk of residual OSA and adenoid regrowth, which occurs in more than 12% of cases within 1.5 years [9].
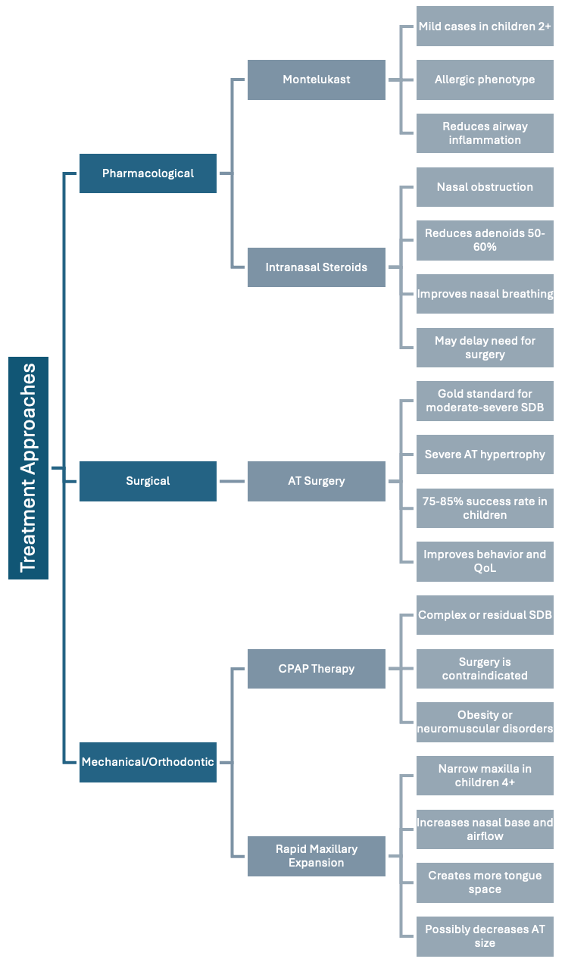
Figure 3. Evidence-Based Treatment Algorithm for Pediatric SDB: A systematic approach to intervention based on severity, age, and associated factors, highlighting the range of options from watchful waiting to surgical and orthodontic interventions.
Long-term Consequences
The implications of untreated pediatric SDB extend far beyond childhood sleep disturbances. Affected children may develop ADHD, learning disabilities, impaired growth, memory deficits, and executive function disorders [2] (Figure 4).
Cardiovascular consequences include ventricular hypertrophy [5], increased oxidative stress [22], and elevated inflammatory markers [24]. The bidirectional relationship with obesity manifests as higher cholesterol levels, insulin resistance, and increased leptin levels that encourage overeating [2, 23].
Even primary snoring which is often considered benign is associated with poorer endothelial function, elevated blood pressure [25], and cognitive issues regardless of AHI severity [26, 27]. This suggests that any degree of SDB warrants clinical attention and appropriate intervention.
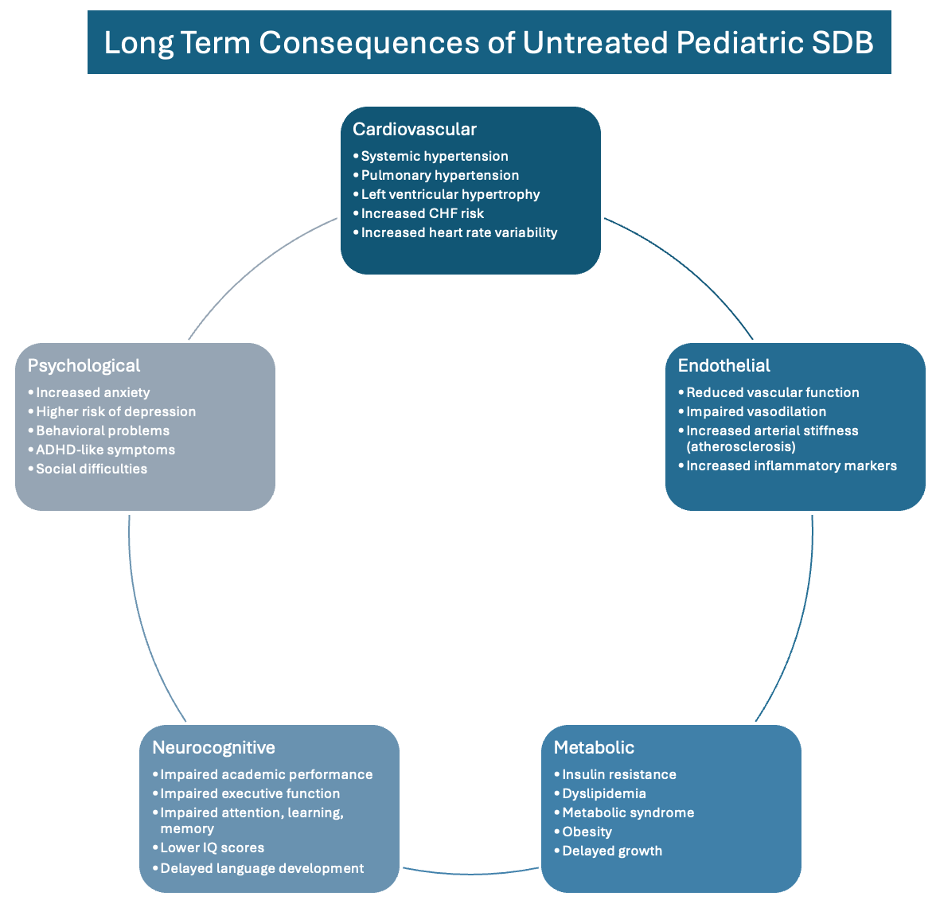
Figure 4. Multisystem Consequences of Untreated Sleep-Disordered Breathing: The cascade of long-term health impacts across cardiovascular, metabolic, neurocognitive, and behavioral domains, illustrating the importance of early identification and intervention.
Conclusion
Untreated childhood sleep-disordered breathing establishes lifelong health risks through mechanisms of chronic intermittent hypoxia and systemic inflammation that increase the likelihood of cardiovascular and metabolic disease. As dental practitioners, we are uniquely positioned to recognize SDB’s clinical manifestations in orofacial structures during routine examinations.
By incorporating targeted screening, recognizing early warning signs, and facilitating appropriate referrals, dentists and healthcare practitioners serve as guardians of systemic well-being beyond oral health. The thorough orofacial examination and intervention may prevent premature cardiovascular mortality and significantly improve the quality of life across our patients’ lifespans.
Earn an Online Postgraduate Degree in Orofacial Pain and Oral Medicine
Are you interested in a variety of issues focused on orofacial pain, medicine and sleep disorders? Consider enrolling in the Herman Ostrow School of Dentistry of USC’s online, competency-based certificate or master’s program in Orofacial Pain and Oral Medicine.
References
- Keefe, K. R., Patel, P. N., & Levi, J. R. (2019). The shifting relationship between weight and pediatric obstructive sleep apnea: A historical review. The Laryngoscope, 129(10), 2414–2419. https://doi.org/10.1002/lary.27606
- Thomas, S., Patel, S., Gummalla, P., Tablizo, M. A., & Kier, C. (2022). You Cannot Hit Snooze on OSA: Sequelae of Pediatric Obstructive Sleep Apnea. Children (Basel, Switzerland), 9(2), 261. https://doi.org/10.3390/children9020261
- Vlahandonis, A., Walter, L. M., & Horne, R. S. (2013). Does treatment of SDB in children improve cardiovascular outcome?. Sleep medicine reviews, 17(1), 75–85. https://doi.org/10.1016/j.smrv.2012.04.004
- Piotto, M., Gambadauro, A., Rocchi, A., Lelii, M., Madini, B., Cerrato, L., Chironi, F., Belhaj, Y., & Patria, M. F. (2023). Pediatric Sleep Respiratory Disorders: A Narrative Review of Epidemiology and Risk Factors. Children (Basel, Switzerland), 10(6), 955. https://doi.org/10.3390/children10060955
- Amin, R. S., Kimball, T. R., Bean, J. A., Jeffries, J. L., Willging, J. P., Cotton, R. T., Witt, S. A., Glascock, B. J., & Daniels, S. R. (2002). Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. American journal of respiratory and critical care medicine, 165(10), 1395–1399. https://doi.org/10.1164/rccm.2105118
- Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Fedok F, Vlasic V, Graff G. Blood pressure associated with sleep-disordered breathing in a population sample of children. Hypertension. 2008 Nov;52(5):841-6. doi: 10.1161/HYPERTENSIONAHA.108.116756. Epub 2008 Oct 6. PMID: 18838624; PMCID: PMC3597109.
- Fagundes, N. C. F., Gianoni-Capenakas, S., Heo, G., & Flores-Mir, C. (2022). Craniofacial features in children with obstructive sleep apnea: a systematic review and meta-analysis. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine, 18(7), 1865–1875. https://doi.org/10.5664/jcsm.9904
- Katyal, V., Pamula, Y., Daynes, C. N., Martin, J., Dreyer, C. W., Kennedy, D., & Sampson, W. J. (2013). Craniofacial and upper airway morphology in pediatric sleep-disordered breathing and changes in quality of life with rapid maxillary expansion. American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics, 144(6), 860–871. https://doi.org/10.1016/j.ajodo.2013.08.015
- Kaditis, A. G., Alonso Alvarez, M. L., Boudewyns, A., Alexopoulos, E. I., Ersu, R., Joosten, K., Larramona, H., Miano, S., Narang, I., Trang, H., Tsaoussoglou, M., Vandenbussche, N., Villa, M. P., Van Waardenburg, D., Weber, S., & Verhulst, S. (2016). Obstructive sleep disordered breathing in 2- to 18-year-old children: diagnosis and management. The European respiratory journal, 47(1), 69–94. https://doi.org/10.1183/13993003.00385-2015
- Chen, L., Huang, J., Jiang, T., Luo, H., Wei, C., Wu, H., Shao, J., & Li, W. (2024). Comparing Sleep Patterns and Clinical Features between Preschool and School-Age Children with OSA. The Laryngoscope, 134(3), 1472–1478. https://doi.org/10.1002/lary.31051
- Beebe, D. W., Ris, M. D., Kramer, M. E., Long, E., & Amin, R. (2010). The association between sleep disordered breathing, academic grades, and cognitive and behavioral functioning among overweight subjects during middle to late childhood. Sleep, 33(11), 1447–1456. https://doi.org/10.1093/sleep/33.11.1447
- Ma, Y., Xie, L., & Wu, W. (2024). The effects of adenoid hypertrophy and oral breathing on maxillofacial development: a review of the literature. The Journal of clinical pediatric dentistry, 48(1), 1–6. https://doi.org/10.22514/jocpd.2024.001
- Galeotti, A., Festa, P., Viarani, V., D’Antò, V., Sitzia, E., Piga, S., & Pavone, M. (2018). Prevalence of malocclusion in children with obstructive sleep apnoea. Orthodontics & craniofacial research, 21(4), 242–247. https://doi.org/10.1111/ocr.12242
- Katyal, V., Pamula, Y., Martin, A. J., Daynes, C. N., Kennedy, J. D., & Sampson, W. J. (2013). Craniofacial and upper airway morphology in pediatric sleep-disordered breathing: Systematic review and meta-analysis. American journal of orthodontics and dentofacial orthopedics : official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics, 143(1), 20–30.e3. https://doi.org/10.1016/j.ajodo.2012.08.021
- Carra, M.C., Huynh, N., Morton, P., Rompré, P.H., Papadakis, A., Remise, C. and Lavigne, G.J. (2011), Prevalence and risk factors of sleep bruxism and wake-time tooth clenching in a 7- to 17-yr-old population. European Journal of Oral Sciences, 119: 386-394. https://doi-org.libproxy1.usc.edu/10.1111/j.1600-0722.2011.00846.x
- Gouthro K, Slowik JM. Pediatric Obstructive Sleep Apnea. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557610/
- Yang, B., Zou, Q., Wang, F., Pang, Y., Wei, P., & Xing, Y. (2024). Allergic rhinitis as a predictor of moderate-to-severe paediatric obstructive sleep apnoea. Sleep & breathing = Schlaf & Atmung, 28(3), 1303–1310. https://doi.org/10.1007/s11325-024-03011-6
- Mitchell, R. B., Archer, S. M., Ishman, S. L., Rosenfeld, R. M., Coles, S., Finestone, S. A., Friedman, N. R., Giordano, T., Hildrew, D. M., Kim, T. W., Lloyd, R. M., Parikh, S. R., Shulman, S. T., Walner, D. L., Walsh, S. A., & Nnacheta, L. C. (2019). Clinical Practice Guideline: Tonsillectomy in Children (Update)-Executive Summary. Otolaryngology–head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery, 160(2), 187–205. https://doi.org/10.1177/0194599818807917
- Ersu, R., Chen, M. L., Ehsan, Z., Ishman, S. L., Redline, S., & Narang, I. (2023). Persistent obstructive sleep apnoea in children: treatment options and management considerations. The Lancet. Respiratory medicine, 11(3), 283–296. https://doi.org/10.1016/S2213-2600(22)00262-4
- Yoon, A., Abdelwahab, M., Bockow, R., Vakili, A., Lovell, K., Chang, I., Ganguly, R., Liu, S. Y., Kushida, C., & Hong, C. (2022). Impact of rapid palatal expansion on the size of adenoids and tonsils in children. Sleep medicine, 92, 96–102. https://doi.org/10.1016/j.sleep.2022.02.011
- Inchingolo, A. D., Laforgia, A., Inchingolo, A. M., Latini, G., Pezzolla, C., Nardelli, P., Palermo, A., Inchingolo, F., Malcangi, G., & Dipalma, G. (2025). Rapid palate expansion’s impact on nasal breathing: A systematic review. International journal of pediatric otorhinolaryngology, 190, 112248. https://doi.org/10.1016/j.ijporl.2025.112248
- Gozal D, Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008 Feb 15;177(4):369-75. doi: 10.1164/rccm.200608-1190PP. Epub 2007 Nov 1. PMID: 17975198; PMCID: PMC2258438.
- Kim, J., Bhattacharjee, R., Kheirandish-Gozal, L., Khalyfa, A., Sans Capdevila, O., Tauman, R., & Gozal, D. (2010). Insulin sensitivity, serum lipids, and systemic inflammatory markers in school-aged obese and nonobese children. International journal of pediatrics, 2010, 846098. https://doi.org/10.1155/2010/846098
- Kheirandish-Gozal L, Gozal D. Pediatric OSA Syndrome Morbidity Biomarkers: The Hunt Is Finally On! Chest. 2017 Feb;151(2):500-506. doi: 10.1016/j.chest.2016.09.026. Epub 2016 Oct 6. PMID: 27720883; PMCID: PMC5310114.
- Au, C. T., Chan, K. C., Chook, P., Wing, Y. K., & Li, A. M. (2021). Cardiovascular risks of children with primary snoring: A 5-year follow-up study. Respirology (Carlton, Vic.), 26(8), 796–803. https://doi.org/10.1111/resp.14089
- Smith, D. L., Gozal, D., Hunter, S. J., & Kheirandish-Gozal, L. (2017). Frequency of snoring, rather than apnea-hypopnea index, predicts both cognitive and behavioral problems in young children. Sleep medicine, 34, 170–178. https://doi.org/10.1016/j.sleep.2017.02.028
- Biggs, S. N., Nixon, G. M., & Horne, R. S. (2014). The conundrum of primary snoring in children: what are we missing in regards to cognitive and behavioural morbidity?. Sleep medicine reviews, 18(6), 463–475. https://doi.org/10.1016/j.smrv.2014.06.009


